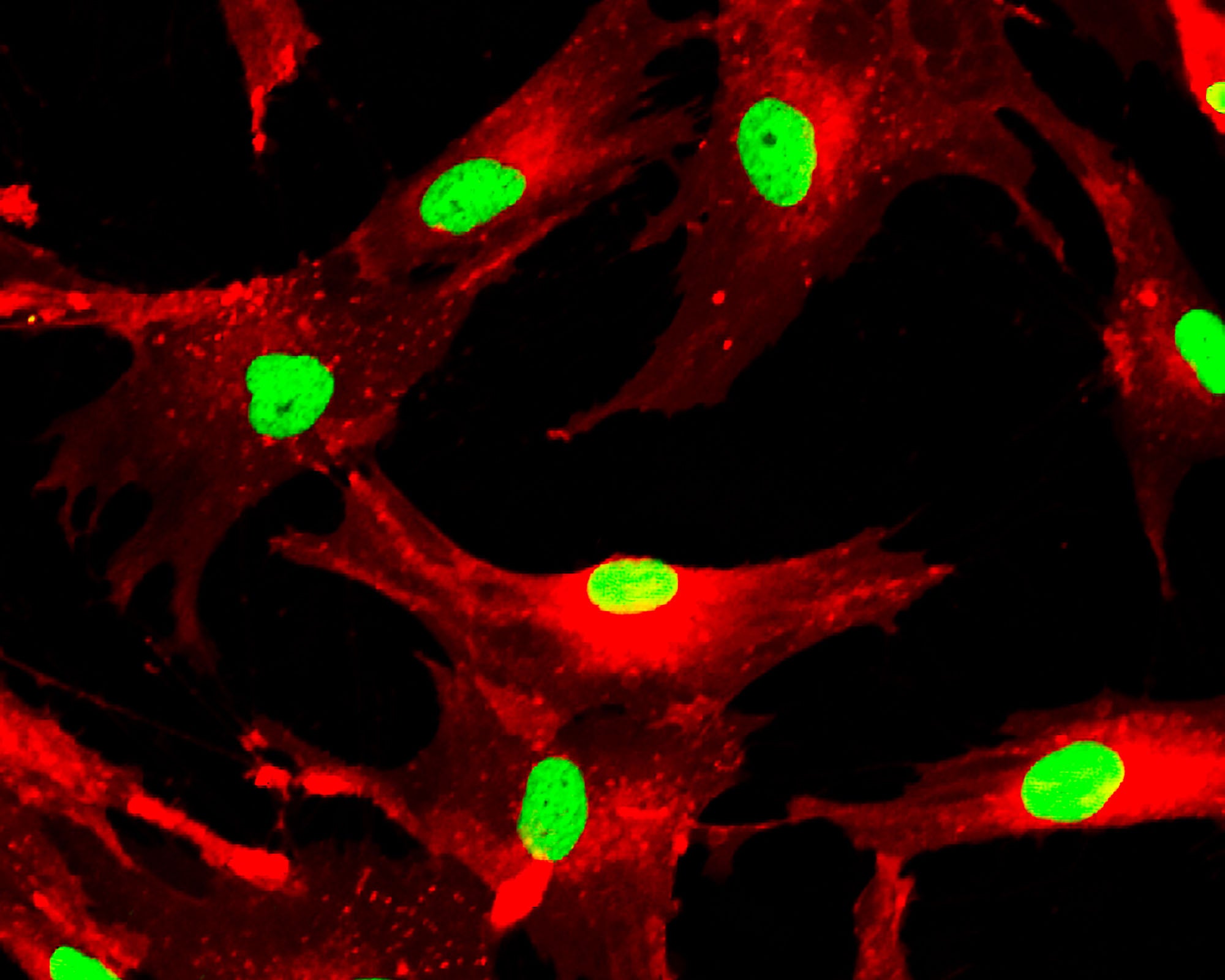
Mesenchymal stem cells (MSCs) have long held therapeutic value due to their self-regeneration, differentiation, and immunomodulation properties. Manufacturing MSCs at scale and under GMP compliance has been a challenge however, largely due to materials such as coating reagents. Here, Terumo Blood and Cell Technologies and Akron Bio have partnered to develop a GMP-compliant MSC manufacturing workflow that is closed, automated, and scalable.
Key Takeaways:
- Full MSC workflow with research and GMP-grade fibronectin
- MSC harvest yields, doubling times, and expression of ISCT flow cytometry standard markers
- Trilineage differentiation results as assessed by MSC morphology



 Back
Back